Systems Pharmacology or Quantitative Systems Pharmacology (QSP) is the field where systems biology approaches are explicitly applied to pharmacology. It is, in a way, an intersection between pharmacometrics and systems biology. The aim is to understand how a ‘system’ responds to a ‘stimulus’. I almost exclusively refer to the human organism as a whole, a particular (human) tissue/organ or a network when talking about a ‘system’ in this blog. Similarly, the stimulus is a specific drug or a drug combination in most cases. So, we can define the aim more specifically as to predict the novel response of the human body, tissue or cell to a certain drug. State-of-the-art models on one of three levels of molecular, cellular and organ networks are utilized depending on the subject at hand. These models can sometimes be integrated, depending on the need and resources. We had briefly discussed the computational requirements for modeling in our first blog. It is crucial to keep the balance between accuracy and computational feasibility while getting the best value out of your data. Yes, very much as in our motto ‘More value out of your data!’.
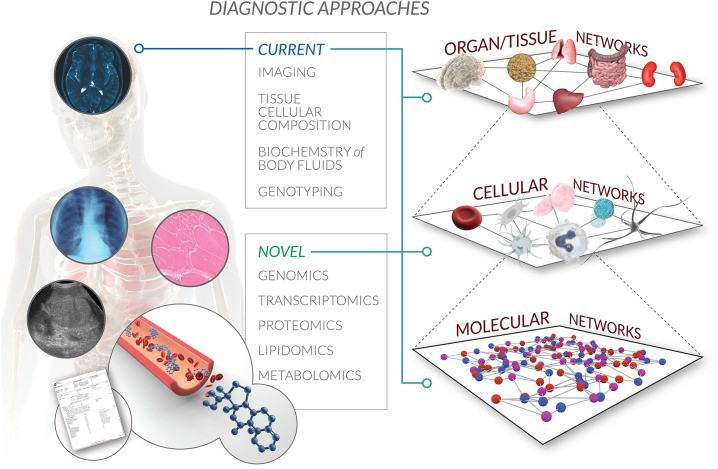
What can the systems biology approach really mean for drug development?
Systems pharmacology approaches can be utilized to predict responses at any stage of drug development, from target identification and optimization to phase 3 clinical trials and drug repurposing. Therefore, it has the potential to transform the drug development process completely. If we go back to how we defined what systems biology does in our previous blog: it uncovers hidden patterns, obtains further insights from existing data, forms hypotheses for downstream research, and makes predictions through simulations to support decision making; then, some examples of what systems pharmacology can be used for will be:
- Patient stratification to ensure higher success rates in treatment and clinical study outcomes by uncovering patterns.
- Predicting response to a novel drug entity based on (pre-)clinical data.
- Identifying possible pathways and most probable targets within the pathways when developing (novel) therapies.
- Evaluating and ranking drug candidates based on their efficacy, efficiency and side effects.
- Improving dosing regimen, clinical trial design and treatment outcome.
One crucial component to a successful implementation of systems pharmacology in any example is the data. Although you will not always need an immense amount of data to adopt systems pharmacology in your drug development process, utilizing data and sometimes specifically omics data can open up new frontiers. Nevertheless, you will need some data, be it omics or clinical data, and the data quality will impact the QSP models.
Systems Pharmacology is an up-and-coming field with a clear potential to improve the drug development process. Systems pharmacology approaches and QSP models are becoming increasingly popular in pharmaceutical research, and for good reasons. We, at PD-value, provide cutting edge systems biology, QSP and pharmacometric modeling to support our clients, not only from a scientific perspective but also from a regulatory perspective. Therefore, in our next blog, we will talk exclusively about the FDA’s increasing focus on systems biology and systems pharmacology.
I hope you enjoyed reading our blog post about systems biology, where we dug a bit deeper than the last time. Follow us for more blog posts like this one and news about us and our field. If you are a pharmaceutical industry researcher who wants to learn more about systems biology, systems pharmacology and their implementation in (pre-)clinical studies, click here to email us to get your consultation.
Next Read: From Nice to Have to Best Practice: How the FDA’s focus on Systems Pharmacology has been increasing
11 January 2022 – Basak Tektemur-Altay
